



Received: 24-May-2021, Manuscript No. GJARR-22-64736(PQ); Editor assigned: 27-May-2022, Pre QC No. GJARR-22-64736; Reviewed: 13-Jun-2022, QC No. GJARR-22-64736(R); Revised: 25-Jul-2022, Manuscript No. GJARR-22-64736; Published: 02-Aug-2022, DOI: 10.15651/GJARR.22.9.6
This study was conducted in order to investigate the morphophysiologycal responses of Frankenia (Frankenia thymifolia Desf.) to water deficit stress and salicylic acid application in 2016, in horticultural science department, at University of Zanjan, Iran. Three soil available water levels (50, 75, and 100 %) and salicylic acid (0, 1 and 2 millimolar) were applied in a factorial experiment based on completely randomized design, with four replications. Water deficit reduced leaves relative water content and shoot growth whereas, root growth, root-shoot ratio, leaves antioxidant capacity, electrolyte leakage, proline, total phenol, and chlorophyll content increased with decreasing soil available water. Salicylic acid application ameliorates the adverse effects of water deficit in Frankenia by increasing leaves antioxidant capacity, proline, and chlorophyll content as well as reducing electrolyte leakage. This effect was more pronounced in 2 millimolars, suggesting that higher concentrations of salicylic acid must be evaluated.
Available water, Chlorophyll, Proline, Shoot growth, Salicylic acid, Total phenol
In recent years, especially in countries with arid and semi-arid climates, the expansion of new green spaces has been severely restricted due to water shortages. Also, water consumption in grasses, not only in arid and semi-arid areas, but also in humid and semi-humid areas, has been a major challenge for green space managers due to reduced rainfall and increasing air temperature (Salahvarzi Y, et al., 2009). Ground covers are short and fast growing plants of low expectation for nutrients and water, which have drawn the researchers’ interest (Al- Tameme HJM, et al., 2016). Frankenia belongs to the Frankeniaceae family. This plant is evergreen and its final height is 7.5 cm. The leaves are small and triangular, arranged along the hairy stems. The flowers are very small, of reddish-pink color, arranged in clusters. It needs full light and grow in most dry and saline soils, and can be used as a cover plant in hot and sunny areas of rock gardens. This plant does not need much irrigation when it is established.
Water deficit stress occurs when the rate of plant water loss due to evapotranspiration, is greater than the water uptake from the soil that can affect many aspects of plant metabolism and growth. Water deficit can affect the process of photosynthesis, respiration, and transpiration through affecting cell inflammation and opening and closing of pores, and on the other hand, has an adverse effect on plant growth by affecting enzymatic processes that are directly controlled by water potential (Mata CG, et al., 2001).
Salicylic acid or ortho hydroxyl benzoic acid is a plant growth regulator of the phenol group and as a nonenzymatic antioxidant agent, and in low concentration, is effective in regulating many physiological processes in plants (Deef HE, et al. 2007). This substance plays an important role in plants exposed to living and non-living stresses, and initial reports indicate that salicylic acid has a regulatory role against a wide range of oxidative stresses in plants (Sakihama Y, et al., 2002).
In a study, the application of salicylic acid with 50, 100, and 150 ppm concentrations, on rice seeds under water deficit stress, increased the plant yield (Farooq M, et al., 2009). It has also been shown that the leaves relative water content, dry weight, chlorophyll, carboxylase, and rubisco activity of salicylic acid-treated wheat under water deficit stress was higher compared to control treatments (Singh B, et al., 2003). The aim of this study was to investigate the morphophysiological traits of Frankenia as affected by salicylic acid, based on previous studies on other plants, to increase its tolerance to water deficit.
In order to investigate the effect of salicylic acid on water deficit tolerance in Frankenia, a study was conducted during the years 2015-2016, in the research greenhouse of horticultural science department, faculty of agriculture, university of Zanjan, Zanjan, Iran. The study was performed as a factorial experiment based on completely randomized design, with four replications. Experiment treatments included three soil available water levels (40, 70, and 100%), and the application of salicylic acid at three levels (0, 1, and 2 mm). For this purpose, Frankenia cuttings were planted in pots, 20 cm high, and 14 cm in diameter, containing loamy sandy soil. After rooting, while receiving sufficient water until the beginning of the treatments, they were fertilized weekly with complete fertilizer (20-20-20). With full establishment of plants after six months and complete coverage of the pot surface, the stress treatments were applied for eight weeks. In order to apply water deficit stress, the soil moisture content in field capacity (31.4% by volume) and permanent wilting point (10.2% by volume) were determined in the soil science laboratory. The amount of available water (20.2% by volume) was obtained from the difference of field capacity and permanent wilting point, and treatments of 100, 70, and 40% of available water were performed. The Theta probe was used to measure soil moisture and calculate its changes between two irrigations. In order to calibrate the device, water (of 100% humidity) and completely dry soil (of zero humidity) were applied. In order to find out the soil moisture content of the pots, the sensors of the device were placed at a depth of 12 cm in the soil during the experiment, and the volumetric soil moisture was recorded. Soil moisture was measured many times and irrigation was done at certain times based on experiment treatments. Salicylic acid was weekly sprayed on the plants in six stages (20 cc per pot). After six weeks of treatments, the desired traits were measured.
To measure shoot growth at the end of the experiment, the aerial parts of the plant were isolated from the roots and dried for 48 hours at 70°C and their dry weight was calculated. In order to calculate the growth rate of roots, after removing the plants from the pot at the end of the experiment, the roots were isolated, and after washing and separating the sand particles, they were dried for 48 hours at 70°C, and their dry weight was calculated. Rootshoot ratio was obtained through dividing the dry weight of roots and shoots.
Proline content was determined according to the method described by Bates et al. using a spectrophotometer (UV-120-20, Japan) at a 520 nm wavelength and the appropriate standards (Bates L, et al., 1973).
In order to calculate electrolyte leakage, leaf samples with distilled water were placed on a shaker for 24 hours and the electrical conductivity was measured. The samples were then placed in an autoclave at 120°C for 20 minutes, and after cooling, their electrical conductivity was measured again. The amount of electrolyte leakage was calculated as a percentage by dividing the initial electrical conductivity and the electrical conductivity of dead cells (Sullivan CY, et al., 1979
Chlorophyll content was measured according to the method of Saini et al. using the formula: mg Chl/g fw=((20.2 (OD 645 nm)+(8.02(OD 663 nm)) × V/(fw × 1000), where OD is optical density, V is the final solution volume in mL and fw is tissue fresh weight in mg (Saini RS, et al., 2001). Relative water content estimation was performed by incubating 0.2 g of fresh leaf sample in 50 mL of distilled water for 4 h. Then the turgid weights of leaf sample were measured. The leaf samples were oven dried at 60°C for 48 h for dry weight calculation at 60°C for 48 h. RWC was calculated by the following equation:
RWC (%)=(fw-dw)/(tw-dw) × 100, where fw, dw, and tw are fresh, dry and turgid weights, respectively (Sairam RK, et al., 2002).
Leaf extraction was performed to measure total phenol and antioxidant capacity using methanol and acetic acid (Bakhshi D, et al., 2006). To calculate the antioxidant capacity, 50 μl of plant extract and 950 μl of 0.1 normal solutions (DPPH) were mixed and kept in a dark chamber at room temperature for 30 minutes. The blank and standard samples consisted of 1 ml of extraction solvent and 1 ml of 0.1 normal solutions (DPPH) respectively. The absorbance was measured in standard and blank samples at 517 nm. The antioxidant capacity of the extracts was calculated as the percentage of inhibition of oxygen free radicals (DPPH) by the following equation:
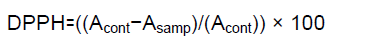
In this equation, DPPH is the percentage of inhibition of free radical, Acont is the rate of absorption of DPPH, and Asamp is the rate of absorption of the extract (Eberhardt MV, et al., 2000).
To measure total phenol, 0.5 ml of plant extract was mixed with 0.5 ml of folin reagent, and 7 ml of distilled water, and placed at room temperature for three minutes; then 1 ml of 20% sodium carbonate was added to the mixture. After an hour, the amount of dye absorption at wavelength of 725 nm was read using a spectrophotometer (Seevers PM, et al., 1970).
Finally, the analysis of variance of the data was performed using SAS software and Duncan's multiple range test (5% probability level) was applied to compare the means. Graphs were drawn using excel software.
The results showed that the lowest root dry weight (3.11 g) was obtained in full irrigation treatment and the highest (8.23 g) was obtained in severe moisture stress. Also, the results showed that salicylic acid increased root dry weight (Figure 1). The interaction effect of water deficit stress and salicylic acid on shoot and root dry weight and root-shoot ratio was not significant (Table 1).
| Source | DF | Root dry weight | Shoot dry weight | Root-shoot ratio | Electrolyte leakage | Chlorophyll | Relative water content | Proline | Total antioxidant | Total phenol |
|---|---|---|---|---|---|---|---|---|---|---|
| Available water | 2 | 35.34** | 4027.22 ** | 0.0823 ** | 2193.84 ** | 6.86** | 1135.05** | 14.54** | 447.22** | 0.000895** |
| Salicylic acid | 2 | 3.12 ** | 204.08 ** | 0.001** | 202.11** | 1.09** | 84.89** | 1.17** | 116.53** | 0.000241** |
| Salicylic acid ×Available water | 4 | 0.374 ns | 71.52 ** | 0.0022 ** | 19.83** | 0.136** | 15.26** | 0.039 ns | 8.72** | 0.000014 ns |
| Error | 27 | 0.146 | 4.22 | 0.000091 | 2.2 | 0.0165 | 4.4 | 0.046 | 1.74 | 5.8 E-06 |
| Coefficient of variation | 6.73 | Mar-77 | 7.71 | 5.72 | 5.04 | 2.68 | 7.76 | 3.86 | 5.75 |
**: Significant at 1% probability level; *: Significant at 5% probability level; ns: Non-significant
In each graph, the averages with a common letter do not show a significant difference at the level of 5% of Duncan’s multiple range tests.
Root weight gain under water deficit conditions has been proposed as one of the most important adaptation mechanisms to improve water uptake efficiency (Huang B, et al., 2000).
The roots have a greater ability to maintain turgor pressure than the leaves, due to osmotic modulation. The difference in susceptibility of shoots and roots in water deficit stress conditions is probably due to the effects of abscisic acid, which inhibits shoot growth while maintaining root growth (Hsiao TC, et al., 2000). During water deficit stress, carbon allocation to the roots, relative to the shoot, increases and helps the roots to survive the water deficit stress.
One of the important effects of salicylic acid is to increase root growth.
Increasing the root growth system and maintaining its health using salicylic acid causes more absorption of water and nutrients, which ultimately, leads to increased plant growth? Foliar application of salicylic acid on soybean stems increased the growth of soybean stems and roots in field and greenhouse conditions (Coronado MAG, et al., 1998).
Based on the results, with increasing stress intensity, shoot dry weight decreased significantly. Increasing the concentration of salicylic acid led to an increase in shoot dry weight. Also, with decreasing available water, the root-shoot ratio increased; however, the effect of salicylic acid on the root-shoot ratio was not significant (Figure 2).
In each graph, the averages with a common letter do not show a significant difference at the level of 5% of Duncan's multiple range test.
Among the physiological effects of water deficit stress on plants is reduced vegetative growth, especially shoot growth, and reduced cell elongation. Decreased leaf area is beneficial in water deficit stress conditions as it leads to reduced transpiration (Mahajan S, et al., 2005). Decreased shoot growth under water deficit stress can be due to the allocation of more photosynthetic materials to the roots by the plant, or due to reduced photosynthetic efficiency (Viera HJ, et al., 1991). Lack of water reduces cell turgor and ultimately, leads to reduced cell growth and development, especially in stems and leaves. As cell growth decreases, organ size is limited, that is why the first noticeable effect of water deficit on the plant is reduction in height or smaller leaf size (Taiz L, et al., 2006).
It has been reported that salicylic acid increases plant growth and, of course, increases shoot dry weight by increasing the activity of antioxidant enzymes that protect the plant against oxidative stress (Fabro G, et al., 2004). Water deficit stress reduces shoot growth and thus, brings it closer to root growth, so the root-shoot ratio will tend to one. In this regard, different plants show different responses to water deficit stress (Loomis RS, et al., 1971). In the present study, it seems that one of the important mechanisms involved in increasing water uptake and preventing water deficit stress is to reduce shoot growth and increase root growth.
The results showed that water deficit stress significantly increased the amount of electrolyte leakage, but treatment with salicylic acid reduced the amount of electrolyte leakage. The highest amount of electrolyte leakage was observed in 50% of available water with zero salicylic acid concentration and the lowest amount of electrolyte leakage was observed in 100% of available water with 2 mm salicylic acid concentration (Figure 3).
The averages with a common letter do not show a significant difference at the level of 5% of Duncan's multiple range test.
Electrolyte leakage is one of the important physiological indicators in the assessment of water deficit stress. Increased electrolyte leakage indicates membrane damage (Jinrong L, et al., 2008). Water deficit stress induces oxidative stress and the production of oxygen free radicals, causing the peroxidation of fatty acids in cell membranes and increasing membrane permeability and electrolyte leakage. These results are consistent with the results of previous studies that have reported increasing electrolyte leakage with increasing stress. The application of salicylic acid in annual and perennial plants under various environmental stresses, in addition to increase in plants resistance to pests and diseases, leads to significant decrease in electrolyte leakage (Sakhabutdinova AR, et al., 2003). It has been reported that salicylic acid protects membranes through affecting polyamines such as putrescine, spermine, spermidine, and forming stable complexes with membranes (Nemeth M, et al., 2002). Based on the results of this study treatment with salicylic acid reduces the level of lipid peroxidation and electrolyte leakage.
Investigating the interaction of salicylic acid and water deficit stress shows that the application of salicylic acid has increased the amount of chlorophyll under stress. The highest amount of chlorophyll was observed in 50% of available water with 2 mm salicylic acid concentration and the lowest amount of chlorophyll was obtained in the control treatment (100% of available water) (Figure 4).
The averages with a common letter do not show a significant difference at the level of 5% of Duncan's multiple range test.
Durability of photosynthesis and maintenance of leaf chlorophyll under stress conditions are among the physiological indicators of stress resistance. There have been different reports on the effect of water deficit stress on leaf chlorophyll content. Increase, decrease, or no change in leaf chlorophyll content under water deficit conditions has been reported according to the type of crop, growth stage, duration of stress period, and severity of water deficit stress (Pessarakli M, et al., 1999). One of the effects of water deficit stress is a decrease in cell division and cell size. In these conditions, the number of chloroplasts per unit area increases and ultimately, leads to an increase in the amount of chlorophyll (Rahman MU, et al., 2004). Under water deficit stress conditions, chlorophyll accumulation increases due to reduced leaf area. However, the plant loses more water due to high transpiration, and as a result, the leaves relative water content and photosynthesis decreases. Increased chlorophyll content due to water deficit stress, in safflower has also been reported (Arab S, et al., 2016). It is possible that water deficit causes chlorophyll to accumulate at the lower leaf area due to reduced leaf area and thus, increases its concentration. If the water deficit stress is not too severe, the chlorophyll content increases that is probably due to the decrease in plant fresh weight. As chlorophyll is expressed in terms of fresh weight, more leaves are used to measure chlorophyll in stressed plants (Arghavni M, et al., 2010).
Salicylic acid treated cells appear to activate the antioxidant system and prevent pigment degradation through synthesizing new proteins, and leads to natural production of photosynthetic pigments (Avancini G, et al., 2003). Since salicylic acid is involved in the improvement of chlorophyll production processes, it is effective on chlorophyll content, which is consistent with the results of this study (Gemes K, et al., 2008).
The results show that the leaves relative water content decreases with the reduction of available water. The highest and lowest leaves relative water content are related to 100% of available water with an average of 85.37% and 50% of available water with an average of 67.11% respectively (Figure 5).
In each graph, the averages with a common letter do not show a significant difference at the level of 5% of Duncan's multiple range test.
The leaves relative water content is an indicator of the amount of water in the plant organs or its freshness, and determines the ability of a plant to retain water under stress. The higher the leaves relative water content, the higher the water retention capacity of the plant. Decreased relative water content occurs due to reduced leaf water potential. In most species, when the water uptake through the roots is equal to the rate of transpiration and the leaves water loss, the leaves relative water content is about 85-95% (Taiz L, et al., 2006). Decrease in relative water content was reported with increasing water deficit stress in different grasses, which is consistent with the results of this experiment (Hatamzadeh A, et al., 2015). Salicylic acid produces phenolic compounds which act as a barrier in cell wall against moisture loss and prevent the reduction of plant water content (Bates L, et al., 1973). The results of this study were consistent with the results of experiments conducted on wheat and barley plants under normal conditions and water deficit stress (Singh B, et al., 2003).
The results showed that the highest accumulation of proline was observed in severe water deficit stress (50% of available water), with 2 mm salicylic acid concentration (Figure 6). The interaction of water deficit stress and salicylic acid on Frankenia proline content was not significant.
The averages with a common letter do not show a significant difference at the level of 5% of Duncan's multiple range test.
When the plant is exposed to water deficit stress, proteins are broken down, resulting in an increase in amino acids and amids. One of these amino acids is proline (Barker DJ, et al., 1993). In response to water deficit stress, certain metabolic processes take place in plants that increase the concentration of pure solutes in the cell and thus, causing water to move to the leaf cells and increasing the turgor pressure. A large number of compounds are synthesized which play a key role in maintaining osmotic balance, membrane protection, and macromolecules; one of the most important of which is proline (Mahajan S, et al., 2005). Proline is effective in reducing light damage to the thylakoid membrane through reducing the production of free oxygen (Chaitanya KV, et al., 2009).
In the present study, salicylic acid treatment increased the amount of proline under water deficit conditions. It seems that salicylic acid can increase biosynthesis and proline accumulation through activating the P5CS2 gene. Also it seems that in water deficit stress, salicylic acid acts as an inducer in proline biosynthesis as it has been reported that salicylic acid stimulates proline synthesis in stressed plants through the biosynthesis of abscisic acid (Shakirova FM, et al., 2007).
The results show that antioxidant activity has an increasing trend from control treatment to severe water deficit stress. The highest amount of total antioxidant obtained in severe water deficit stress with 2 mm salicylic acid concentration (Figure 7).
The averages with a common letter do not show a significant difference at the level of 5% of Duncan's multiple range test.
In order to prevent oxidative stress in water deficit stress conditions, the antioxidant activity of the plant increases (Hatamzadeh, et al., (2015). Salicylic acid acts as a growth regulator in plants and can neutralize oxidative stresses resulting from water deficit stress.
The results showed that the highest amount of total phenol was observed under severe water deficit stress. Salicylic acid treatment increased the amount of these compounds (Figure 8). The interaction of water deficit stress and salicylic acid on leaf total phenol content was not significant.
In each graph, the averages with a common letter do not show a significant difference at the level of 5% of Duncan's multiple range test.
It has been reported that phenolic compounds can act as antioxidants in cells through giving up electrons to peroxidase enzymes and detoxification of produced oxygenated water. The increase in phenolic compounds is probably due to their antioxidant role against reactive oxygen species. Many phenolic compounds are highly effective hydrogen peroxide purifiers, hydroxyl radicals, and peroxyl and therefore, can inhibit the lipid peroxidation chain and stabilize membranes.
In general, regarding the effect of salicylic acid in reducing electrolyte leakage and maintaining the leaves relative water content, and increasing the amount of chlorophyll, proline, antioxidant capacity, total phenol, and dry weight of roots and shoots, it can be suggested that the application of this substance in Frankenia reduces the effects of water deficit stress. Based on the results of this study, the application of 2 mm salicylic acid yielded a better result than 1 mm, and it is possible that concentrations higher than 2 mm are more useful. Obviously, the volume of solution consumed per unit area must also be considered.