


Received: 01-Nov-2022, Manuscript No. GJCMB-22-83148; Editor assigned: 04-Nov-2022, Pre QC No. GJCMB-22-83148(PQ); Reviewed: 18-Nov-2022, QC No. GJCMB-22-83148; Revised: 25-Nov-2022, Manuscript No. GJCMB-22-83148(R); Published: 02-Dec-2022, DOI: 10.15651/gjcmb.22.10.012
The genome of an original cell was initially stored in a nucleus that was rather simple. There is no evidence that RNA-world cells ever established nucleate structures or a membrane enclosing their genomes. Everything would have been feasible when one ribozyme RNA replicase emerged, including the creation of DNA polymerases with lipid synthesizing capabilities. The stabilizing choice of RNA-lipid affinities might have then been followed by free energy- or enthalpy-favored processes, and so forth. In the past ten years on the interaction of lipid with RNA has developed in the field of chemical biology, but it is yet unknown how important these studies are for understanding prebiotic processes of the cells. Regarding the origin of eukaryotes and the DNA world, a key idea is that a prokaryotic cell creature was invaded by a different, non-nucleated cell, establishing an endosymbiotic connection and seeding the nuclear envelope with material from the invading organism's outer membrane.
Despite the confusion surrounding its evolutionary history, the nuclear envelope, a bilayer membrane that in many cells is connected to the endoplasmic reticulum, surrounds the nucleus. The regularly observed closeness between the nuclear envelope and the endoplasmic reticulum is repeatedly gone unappreciated, especially as it relates to nuclear isolation and concerns with the purity of the resultant nuclei. It’s possible that the science of cell division is currently concentrating on chromosome capture via kinetochore-microtubule interactions, the mechanics of anaphase, and the functioning of mitotic checkpoints. However, it's also likely that this concentration is omitting one of the fascinating phenomena of all: the development of daughter nuclei. Nuclear lamina, nuclear envelope, and nucleolus precursors develop, mature, and assemble in "daughter to be" cells beginning in anaphase and conclusively in telophase. Nuclear membrane proteins' potential role as integrator for nuclear and cytoplasmic structure and dynamics is also gaining attention.
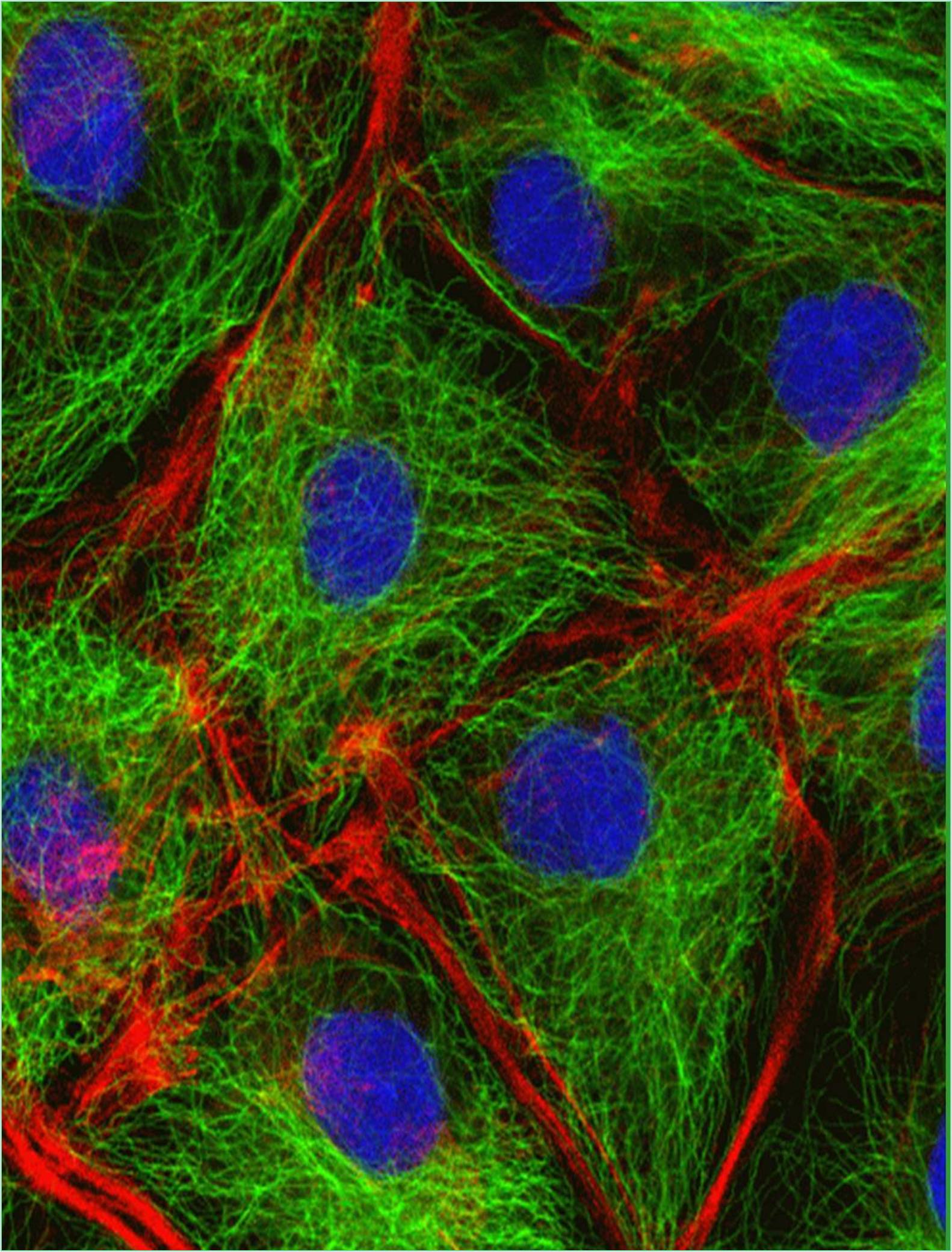
In eukaryotic cells, the nuclear envelope encases the cell nucleus and is made up of a dual membrane, nuclear pore complexes, and the lamina. The inner nuclear membrane of the double membrane enclosing the nucleoplasm has a special collection of membrane proteins that interact with chromatin or the lamina, while the outer nuclear membrane is consistent with the endoplasmic reticulum. The nuclear envelope permeability, or perinuclear space, which serves as a calcium storage area like the endoplasmic reticulum, separates the outside nuclear membrane from the inner nuclear membrane.
The nuclear membrane is fused at nuclear pore complexes involved in nucleocytoplasmic transport and gene control. Additionally, they are linked together via the cytoskeleton and nucleoskeleton. Silent chromatin has been referred to as being located near the nuclear envelope and frequently interacting with the nuclear membrane, whereas active chromatin is found inside the nucleus or interacts with nuclear pore proteins. The key structure of the nuclear lamina, which supports the nuclear envelope, connects chromatin regions to the nuclear peripheral, and localizes some proteins to the nuclear envelope in multicellular cells, is mostly made up of lamins and the proteins that interact with them. Additionally, it has been demonstrated that lamins control a wide range of nuclear functions, such as replication of DNA, transcription, and nucleosome architecture. Some transcripts can be active or triggered at the nuclear lamina, despite lamins preferentially interacting with transcriptionally quiet chromatin. Various cell types have different lamina-associated chromatin domains, which suggest that these domains shift spatiotemporally.
Lamins interact with histones, chromatin-associated proteins, and transposable elements, such as the lamin B receptor, heterochromatin protein 1, barrier to auto integration factor, and oligomer transcription factor 1, in addition to directly interacting with chromatin. Laminopathies, a collection of phenotypically heterogeneous genetic illnesses with signs ranging from neuromuscular disorders and neurotoxicity to premature ageing syndromes, have been discovered to be caused by mutation in lamins or the associated proteins. A-type and B-type nuclear lamins, which are thought to be the evolutionary forerunners of an intermediate filament superfamily, are divided into two distinct classes. The primary lamins of the A-type that are produced by a single gene, lamins, are lamin A, lamin C. In humans and other mammals, the B-type lamins B1, B2, and B3 are encoded by lamins1 and lamins2, respectively.